Physics Timeline
ANTIQUITY
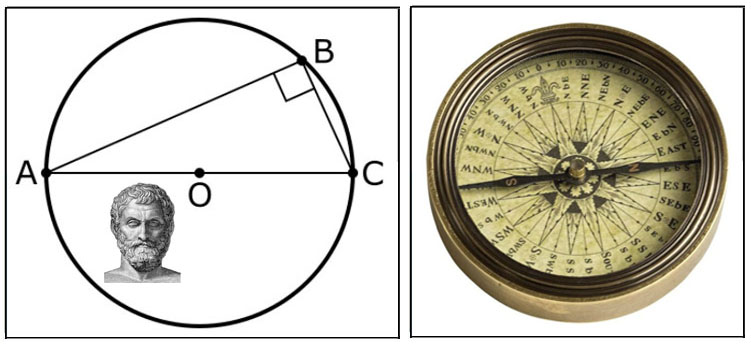
6th century BCE
- Thales's Theorem
A line through the diameter a circle (AC) and any point on the circle (B) will form a right angle (special case of
the 31th proposition of Euclid's Elements). Thales of Miletus (626-545BCE), a pre-Socratic Greek Philosopher, is
noted for breaking away from mythology to explain the world by using mathematics, science (natural philosophy) and
deductive reasoning to explain the world. Thales's view that all of nature is based on the existence of a single
ultimate substance (water) is an early expression of 'first principle' (arche) - the a priori effort of decomposing
things down to fundamental axioms or postulates. One of the earliest known reference to lodestone's magnetic
properties was made by Thales which later led to the development of compasses using magnetic
needles.
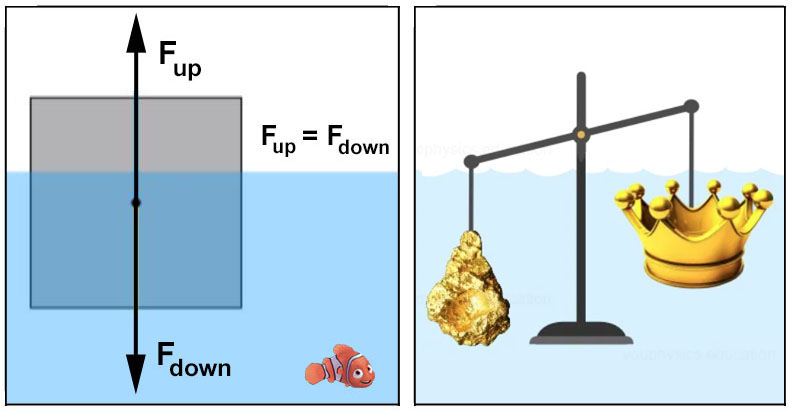
C 245 BCE - Archimedes' Principle
Any object, totally or partially immersed in a fluid or liquid, is buoyed up by a force equal
to the weight of the fluid displaced by the object. King Hiero II of Syracuse suspected his crown was not of pure
gold. Archimedes, while taking a bath and noticing the rising water level, determined that he could determine the
crown's volume (and if silver was mixed in with the gold). According to legend, Archimedes jump out of the
bathtub and ran down the streets crying 'Eureka!' - "I have found it!".
11th CENTURY
1021 - Theory of Vision
Alhazen Hasan Ibn al-Haytham (965-1040) was the first to correctly explain the theory of vision
('Book of Vision'). Alhazen's publications were frequently citied by Isaac Newton Johannes Kepler Christiaan
Huygens and Galileo Galilei. He is known as the 'father of modern optics',
16th
CENTURY
1543 - Heliocentrism
Nicolaus Copernicus (1473-1543) develops the model of the solar system where the Sun is its center
("De revolutionibus orbium coelestium" - "On the Revolutions of the Celestial Spheres", 1543). Major event in
the history of science, triggering the Copernican Revolutions and pioneering the way to the Scientific
Revolution.
1589 - Leaning Tower of Pisa
Experiment
Galileo Galilei (1564–1642) dropped two spheres of different
masses from the Leaning Tower of Pisa to demonstrate that their time of descent was independent of their
mass.
17th
CENTURY
1600 - Electroscope
British physician William Gilbert (1544-1603) invented the electroscope, the first
electrical measuring instrument (to detect the presence of electric charge on a
body).
1609 - Kepler's Laws of
Planetary Motion
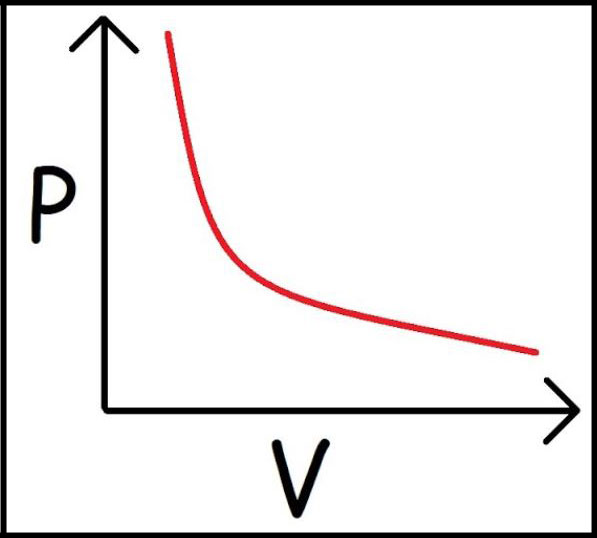
1662 - Boyle's Law
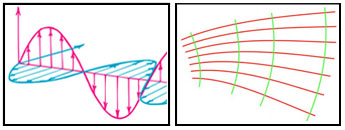
1678 – Huygens's Principle
Christiaan Huygens (1629-1695) proposed that light was made up of waves vibrating up and
down perpendicular to the direction of the wave propagation. Huygens's Principle proposed that every point reached
by a luminous disturbance becomes a source of a spherical wave. The sum of these secondary waves determines the
form of the wave at any subsequent time. In 1704 Sir Isaac Newton advanced the particle theory of light ('Opticks')
where light is made of up of small discrete particles - forerunner to the modern understanding of the
photon.

1687 - Isaac
Newton's (1642–1727) Laws of Motion & Universal
Gravitation
1st Law: an object
at rest will stay at rest. An object in motion will stay in motion unless acted on by a net external
force.
2nd Law: the
rate of change of momentum (mv) of a body over time is directly proportional to the force applied. Constant mass:
F=ma (mass times acceleration). The important point is that Newton told the world that a constant force produces a constant
acceleration. Not a constant velocity – that was the big surprise.
3rd Law: all forces
between two objects exist in equal magnitude and opposite direction. "For every action, there is an equal and
opposite reaction".
Universal Gravitation Law: every particle attracts every other particle in the universe with a force that is directly
proportional to the product of their masses and inversely proportional to the square of the distance between their
centers.
18th CENTURY

1745 – Leyden Jar
The ‘Leyden Jar’ independently discovered by
German cleric Ewald Georg von Kleist (1700-1748) and by Dutch scientist Pieter van Musschenbroek of Leiden
(1692-1761), Netherlands. The ‘Kleistian’ or ‘Leyden’ Jar, the original form of a capacitor, or condenser, consists
of a glass jar with metal foil on the inside and outside surfaces. The Leyden Jar was the first means of storing
electric charge in large quantities which greatly expanded early electrical research. Over a half-century later the
'voltaic pile' was invented (1799).
“The Structure of
Scientific Revolutions” (Thomas Kuhn, 1962): “The Leyden jar belongs to a class that may be described as
theory-induced…Not all theories are paradigm theories. Both during pre-paradigm periods and during the crises
that lead to large-scale changes of paradigm, scientists usually develop many speculative and unarticulated
theories that can themselves point the way to discovery…Only as experiment and tentative theory are together
articulated to match does the discovery emerge and the theory becomes a paradigm. The discovery of the Leyden Jar
displays all these features. When it began there was no single paradigm for electrical research. One of the
competing schools of electricians took electricity to be a fluid and that conception led a number of men to attempt
bottling the fluid by holding a water-filled glass vial in their hands and touching the water to a conductor
suspended from an active electrostatic generator. The initial attempts to store electrical fluid worked only
because investigators held the vial in their hands while stand upon the ground. Electricians still had to
learn that the jar required an outer as well as an inner conducting coating and that the fluid is not really stored
in the jar at all.”
1782 - Conservation of Matter
Antoine Lavoisier (1743-1794) demonstrated the principle of conservation of mass with experiments of
the combustion of masses. Influences John Dalton’s on discovery of the law of multiple proportions regarding
elements and later atomic theory of matter.

1799 – Electric
Battery
Alessandro Volta (1745-1827) invents the 'voltaic pile'. Volta proves that electricity cold be generated chemically
and debunked the theory that electricity was generated solely by living beings. The SI unit of electric potential
is named in his honor as the volt.
19th CENTURY
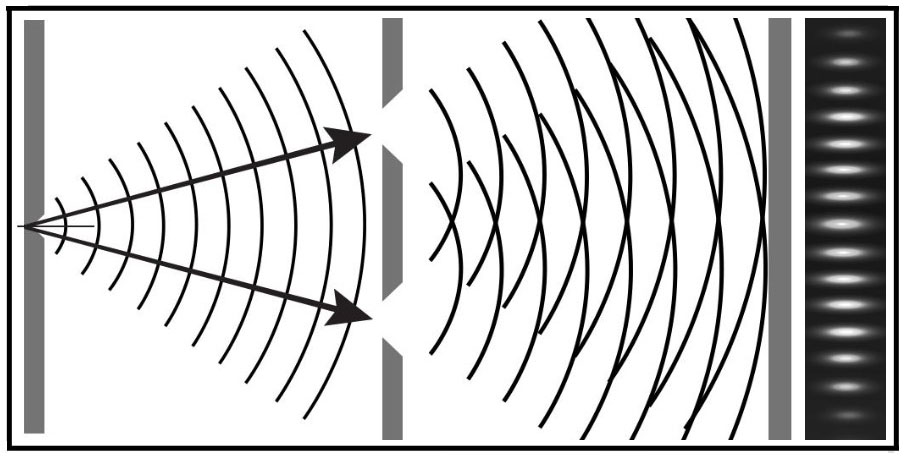
1801 - Double-Slit Experiment
Thomas Young (1773-1829) establishes the wave theory of light with the double-slit experiment.. His
work influenced that of William Herschel,
Hermann von Helmholtz, James Clerk Maxwell and Albert Einstein.
1803 - Atomic Theory of Matter
John Dalton (1766–1844) observed that chemical substances seemed to combine and break down into other
substances by weight in proportions that suggested that each chemical element is ultimately made up of tiny
indivisible particles of consistent weight. The amazing upshot: elements only exist in discrete packets of
matter! ('quantization' was later to be observed with energy - see "1900-Black-body
Radiation Law”).
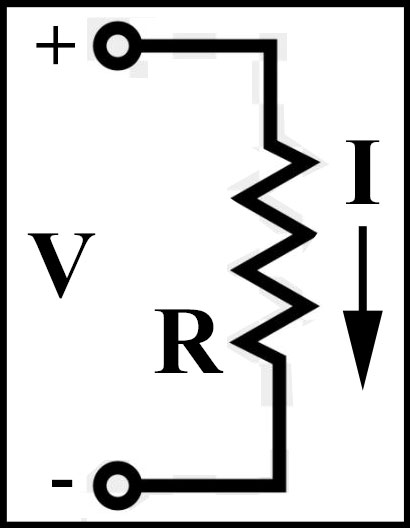
1827 - Electrical
Resistance
Georg Simon Ohm (1789-1854) develops Ohm's law: the potential
difference (voltage) applied across a conductor is proportional to the resultant current
(E=IR).
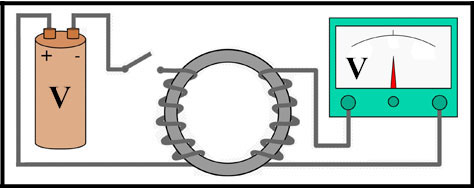
1831 - Faraday's Law of Induction
Michael Faraday's (1791–1867) Law of Electromagnetism predicts how a magnetic field will interact
with an electric circuit to product an electromotive force
(voltage).
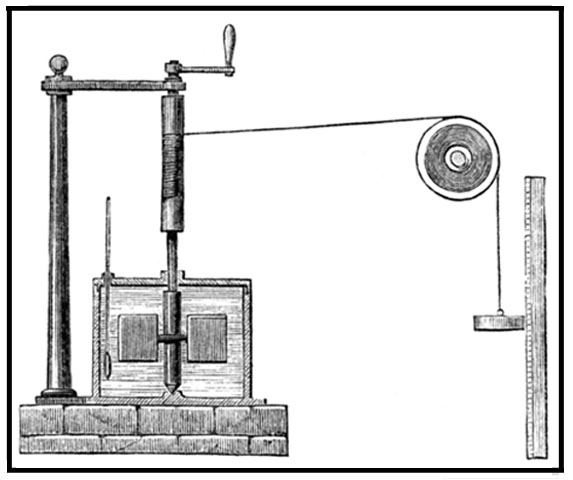
1843 - Mechanical Equivalent of
Heat
James Prescott Joule’s (1818-1889) best-known experiment to determine
the mechanical equivalent of heat involved the use of a falling weight which spun a paddle wheel in an
insulated barrel of water which increased the temperature. After further experimental refinements Joule
declared that 1 BTU is equivalent to 778 foot-pounds of work (3,412kWh). Joule's work led to the law of
conservation of energy which in turn led to the development of the first law of thermodynamics. Understanding
the underpinnings of Joule's work requires one to believe that the collisions of molecules are perfectly
elastic. The scientific community's slow acceptance to Joule's ground breaking idea, which challenged the
Caloric theory, now obsolete, was that the very existence of atoms and molecules was not widely accepted for
another 50 years. The SI unit of energy, the Joule (J), is named after
him.
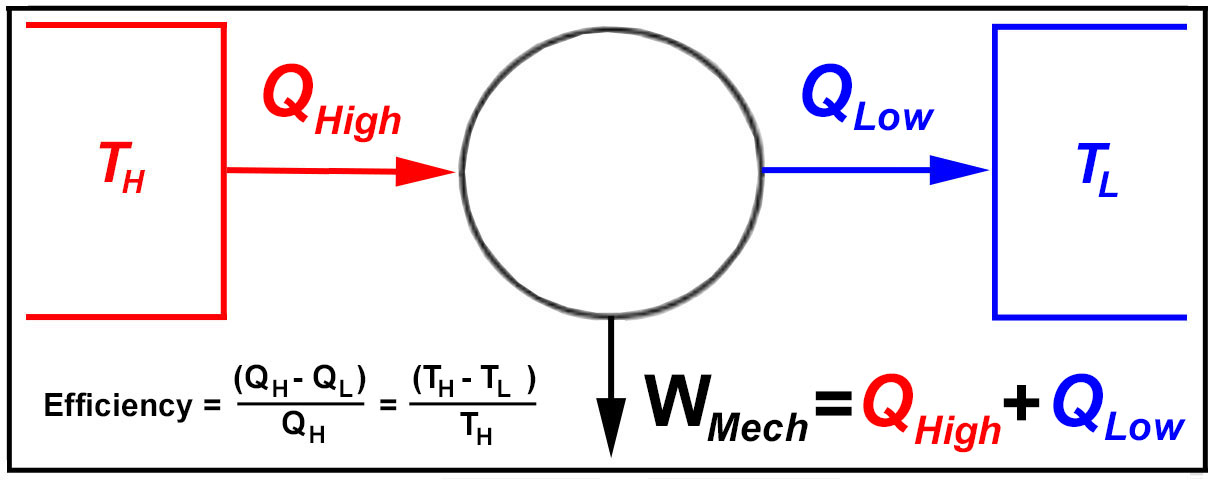
1850 - Clausius
Formalizes the 2nd Law of Thermodynamics
The German scientist Rudolf Clausius (1822-1888) laid the foundation for the second law of thermodynamics in 1850
with the publication of "On the Moving Force of Heat" (1850). Clausius restated Sadi Carnot's principle
(Carnot Cycle) by providing a truer and sounder explanation of the relation between heat transfer and work: "Heat
can never pass from a colder to a warmer body without some other change, connected therewith, occurring at the same
time". In 1865 Clausius introduced the concept of entropy.
1) Heat cannot flow from a cold source to a hotter source, unless work is provided.
2) 100% conversion of heat into mechanical work is not possible.
3) No process is possible in which the entropy decreases. (entropy relates to the 'quality' of heat).
4) There is no principle of conservation of entropy (unlike energy, linear and angular
momentum).
1861 - Maxwell's Equations
Partial differential (space-time) equations that form the foundation of classical
electromagnetism. The equations provide a mathematical model for electric, optical and radio technologies. They
describe how electric and magnetic fields are generated by charges, currents and changes of the fields. The
equations are named after James Clerk Maxwell (1831–1879) who published them, including the Lorentz force law, in
1861 and 1862. The important consequence of Maxwell's equations is that they demonstrate how fluctuating electric
and magnetic currents propagate at a constant speed (c) in a vacuum - known as electromagnetic
radiation.
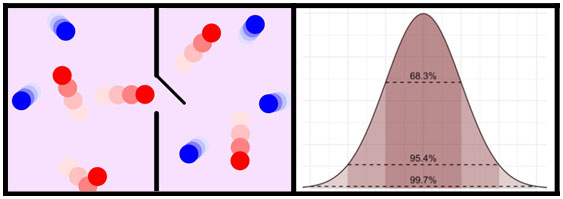
1887 - Statistical Mechanics
Ludwig Boltzmann (1844–1906) develops statistical mechanics to describe how macroscopic
observations of temperature or pressure are correlated to microscopic parameters that fluctuate around an average.
Boltzman also provides the definition of entropy: S = kBln(W) (kB = Boltzmann’s constant = 1.308 x 10-23 J-K-1).
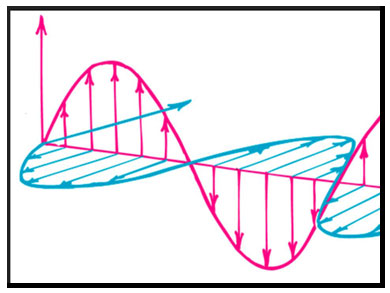
1887 - Electromagnetic Waves
Heinrich Rudolf Hertz (1857-1894) proves the existence of electromagnetic waves predicted by James
Clerk Maxwell's equations of electromagnetism. The unit of frequency, cycle per second, is named the "hertz"
in his honor.
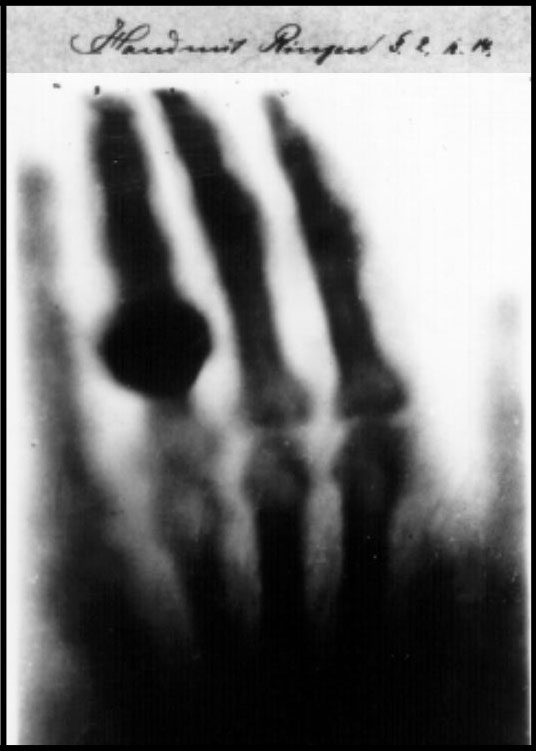
1895 - X-Rays
Wilhelm
Rontgen (1845-1923) produced and detected electromagnetic radiation in a wavelength range known as X-rays
(Rontgen rays). An achievement that earned him the Nobel Prize in Physics (1901).
X-rays are a classic case of discovery through accident – an event that occurs more frequently than the layman
realizes (and where the impersonal standards of scientific reporting reluctant to disclose). During a normal
investigation of cathode rays Roentgen noticed that the barium platino-cyanide screen at some distance from his
shielded apparatus glowed when the discharge was in process. The cause of the glow came in straight lines from the
cathode ray tube, that the radiation cast shadows and could not be deflected by a magnet. The effect was not due to
the cathode rays but to an agent with some similarity to light. X-rays were greeted not only with surprise but with
shock. Lord Kelvin at first pronounced them an elaborate hoax. X-rays were not prohibited by theory,
however they violated deeply entrenched expectations based on established laboratory procedures. X-rays
opened up a new field of scientific investigation and its discovery not only expanded the domain of normal science
but revealed the importance of how anomalies can seriously challenge existing scientific achievements or
‘paradigms’ in the words of Thomas Kuhn (“The Structure of Scientific Revolutions”,1962). Rontgen discovered
x-rays’ medical use when he took a picture of his wife's hand. When she saw the picture, she said "I have seen my
death."
1896 – Discovery
of Radioactivity
After learning about Rontgen's x-ray discovery, Antoine Henri Becquerel (1852-1908) investigated whether if
phosphorescence material emitted x-rays (it did not). In an experiment with non-phosphorescent uranium salts
Becquerel discovered that the salts emitted radiation. Becquerel shared the 1903 Nobel Prize in Physics with Marie
Curie and Pierre Curie for his discovery of radioactivity.
1897 - Electron Discovered
Sir Joseph John Thomson (1856-1940) discovers the first subatomic 'particle' - the electron. Thomson
showed that cathode rays were composed of previously unknow negatively charged particles
(electrons).
20th
CENTURY
1900 - Plank's
Blackbody Radiation Law
Max Karl Ernst
Ludwig Plank (1858–1947) modified Wien's radiation law to develop a mathematical expression (formalism) for
black-body radiation. Unlike the Wien approximation, Planck's law accurately describes the complete spectrum of
thermal radiation. Plank's formalism also relied on Boltzmann's statistical interpretation of the second law of
thermodynamics which led to the Plank's Postulate - that the energy of oscillators in a black body is quantized
(E=nhv; n = integer, h = Plank's constant, v = oscillator frequency). hv is referred to as the energy of quanta
photons. Quantization of energy later gave birth to quantum physics. For the discovery of energy quanta Plank won
the Nobel Prize in Physics (1818).
1905 - Einstein Proves that Atoms
Exist
Albert Einstein (1879–1955) publishes a paper where he modeled the
motion of the pollen particles as being moved by individual water molecules. The formalization of the
Brownian motion served as convincing evidence that atoms and molecules exist.
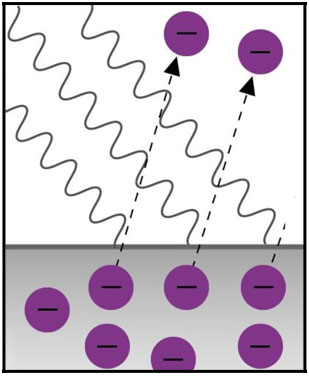
1905 - Einstein's Photoelectric
Effect
Classical electromagnetism predicts that continuous light waves
transfer energy to electrons and that the energy should be proportional to the intensity of the light,
regardless of the light’s color. Electric currents are generated in metals (or particles emitted) when they
are illuminated by blue or ultraviolet light (photoelectric effect). Physicists were surprised to learn that
a red or green light, even a bright beam of red or green light, failed to generate this effect. Albert
Einstein created a new definition of light - that light is made of individual quantum particles. He proposed
that a beam of light is not a propagating wave, but a swarm of discrete energy packets, known as photons, and
that electrons are dislodged only as a function of the light's frequency and not its intensity. Einstein’s
new model (‘truth’) of light is
controversial – the dawn of the wave-particle duality: light is both a wave and a particle. To this day
physicists have failed to devise an experiment to catch light’s true nature. If an experiment is set up to
measure light’s wave properties, light behaves as a wave. If the experiment is set up to measure light’s
particle properties, light behaves as particles. Einstein was awarded the Nobel Prize in Physics (1921) for
discovery of the law of the Photoelectric Effect.
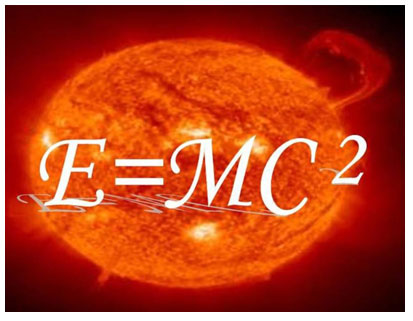
1905 - Einstein's Mass-Energy
Equivalence
Albert Einstein accepted Maxwell’s postulate
that the speed of light is a constant. His grand insight was that time and mass must change as you approached the
speed of light (1905). Albert Einstein's develops the famous formula for mass-energy equivalence:
E=mc2. The energy of a
particle in its rest frame is the product of mass and the speed of light squared. The Space-Time concept is born.
During Einstein’s time people assumed that time was like a watch on God’s hand – that it beat at a steady rate
throughout the universe, no matter where you were. Einstein disagreed with classical physics. The tick, tick of the
wristwatch is actually the click, click of electricity turning into magnetism, turning back into electricity – the
steady pace of light itself. When you approach the speed of light the energy that’s contributing to speed gets put
into mass (since c is constant). Mass gets heavier. The equivalence principle implies that when energy is lost in
chemical reactions, nuclear reactions and other energy transformations, the system will also lose a corresponding
amount of mass. The energy, and mass, can be released to the environment as radiant energy (light) or as thermal
energy.
The scientific community was very slow to respond this radical idea
(‘truth’). Max Planck recognizes Einstein pioneering work and is appointed professor in Zurich University.
Einstein becomes the ‘father’ of modern physics’.
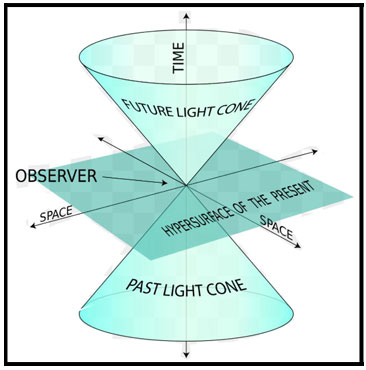
1905 - Einstein's Special Relativity
Special relativity was proposed by Albert Einstein in a 1905 paper titled "On the
Electrodynamics of Moving Bodies". Einstein reasoned that the hypothesized luminiferous aether, the postulated
medium for the propagation of light, could not exist due to the incompatibility of Newtonian mechanics with
Maxwell's equations of electromagnetism (the negative outcome of the Michelson–Morley experiment (1887) suggested
that the aether did not exist). Einstein's development of special relativity corrected the mechanics to handle
situations involving all motions and especially those at a speed close to that of light (known as relativistic
velocities). Today, special relativity is proven to be the most accurate model of motion at any speed when
gravitational and quantum effects are negligible.
1911 - Cloud Chamber
Charles Wilson (1869-1959) invented the cloud chamber (or Wilson Chamber) from researching the
effects of the Brocken spectre (shadow of an observer cast in mid air upon any type of cloud opposite a strong
light source). Refinement of the design permitted the chamber to be a particle detector for visualizing the passage
of ionizing radiation. Wilson received half the Nobel Prize in Physics in 1927 for his work on the cloud
chamber.

1912 - Discovery of Superconductivity
By using liquid helium Dutch experimental physicist Heike Onnes (1853-1926) discovered that the
electrical resistance of solid mercury vanishes at 4.2K. Onnes was awarded the Nobel Prize in Physics in
1913.
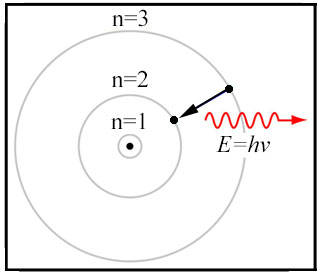
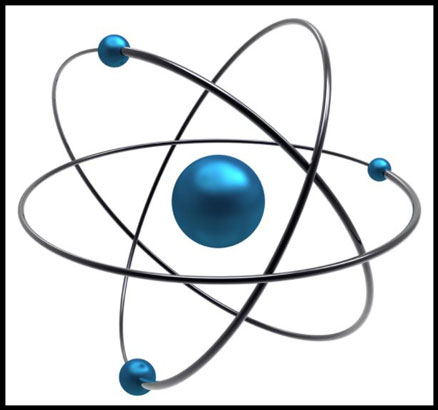
1913 – Bohr’s Model of the
Atom
Niels Bohr (1885–1962) and Ernest Rutherford (1871–1937) develops the "Rutherford-Bohr" atomic model. A 'planetary'
model that consisted of a small, dense nucleus surrounded by orbiting electrons (electrostatic forces took the
place of gravity). The Rutherford-Bohr model typically is called the Bohr model for short. The
Bohr model gave a successful theoretical underpinning of the Rydberg formula which calculated the wavelengths of
hydrogen spectral series. Today the Bohr model is considered an obsolete scientific theory. Besides providing
an adequate first-order approximation of the hydrogen atom, the Bohr model is commonly taught to introduce students
to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence
shell atom. Bohr was awarded the Nobel Prize in Physics (1922) for his investigation of the structure of atoms and
of the radiation emanating from them.
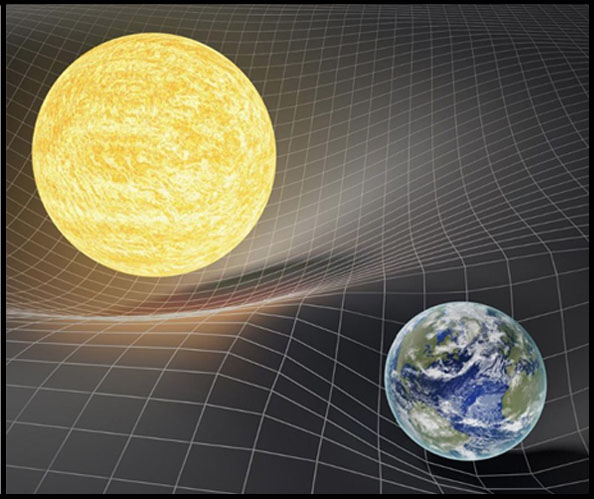
1915 - General Relativity
Albert Einstein publishes "Einstein's theory of gravity", known as the general theory of relativity, which is the
current description of gravitation in modern physics. General relativity provides a unified description of gravity
as a geometric property of space and time, or four-dimensional spacetime, where the curvature of spacetime is
directly related to the energy and momentum of whatever is present, including matter and radiation. General
relativity has held up well to various tests scientists have thrown at it, however it is at a lost to explain the
physics inside a black hole.
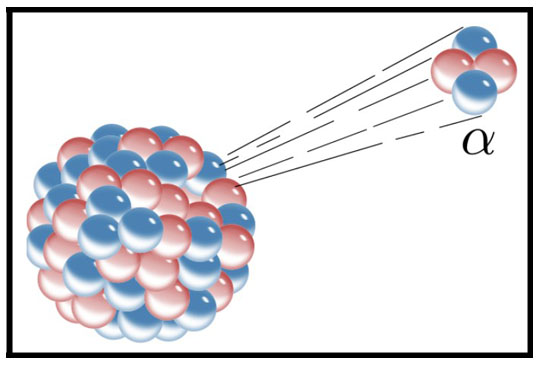
1920 – Rutherford
Isolates the Proton
Initially
Ernest Rutherford only knew about electrons and the positively charged nucleus. When he shot heavy alpha particles
through very thin gold foil, he was astonished to find that a small fraction of particles ricocheted back 180
degrees – as if they had hit a brick wall. Thomson’s plum pudding model could not explain this (where negatively
charged electrons were sprinkled like prunes through a sponge dough of positive charge). Rutherford assumes that
the nucleus was made up of a mix of protons – positively charged particles that he discovered (1918) by isolated
the nuclei of hydrogen. Hydrogen contains just one proton and one electron orbiting it. In 1919 he discovered the
emission of a subatomic particle which he called the "hydrogen atom" but, in 1920, he more accurately named the
proton.
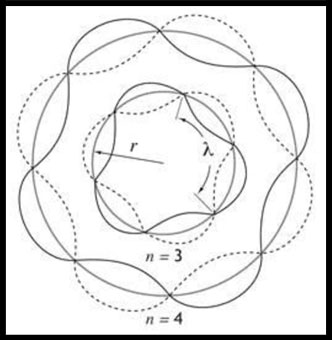
1924 - DeBrogle Waves
In his PhD thesis French physicist Louis de Broglie (1892–1987) proposed that just as
light has both wave-like and particle-like properties, electrons also have wave-like properties. Wave-like behavior
of matter has been confirmed with various metal diffraction experiments using electrons and experiments using other
elementary particles.
1925-1927 - Quantum Mechanics
Quantum mechanics arose gradually from theories to explain observations which could
not be reconciled with classical physics, such as Max Planck's 'quantum oscillator' solution in the black-body
radiation problem and Albert Einstein's observation between energy and frequency in the photoelectric effect. The
modern development of quantum mechanics began in the mid-1920s by Niels Bohr, Werner
Heisenberg, Erwin
Schrodinger, Max Born and others.
Since its inception, the many counter-intuitive aspects and results of quantum
mechanics have provoked strong philosophical debates and many interpretations. The arguments center on the
probabilistic nature of quantum mechanics, the difficulties with wavefunction collapse and the related measurement
problem, and quantum nonlocality. Theoretical physicists Richard Feynman once said, "I think I can safely say that
nobody understands quantum mechanics." According to Steven Weinberg, theoretical physicist and Nobel laureate in
Physics, "There is now in my opinion no entirely satisfactory interpretation of quantum mechanics." The views
of Niels Bohr, Werner Heisenberg and other physicists are often grouped together as the "Copenhagen
interpretation".
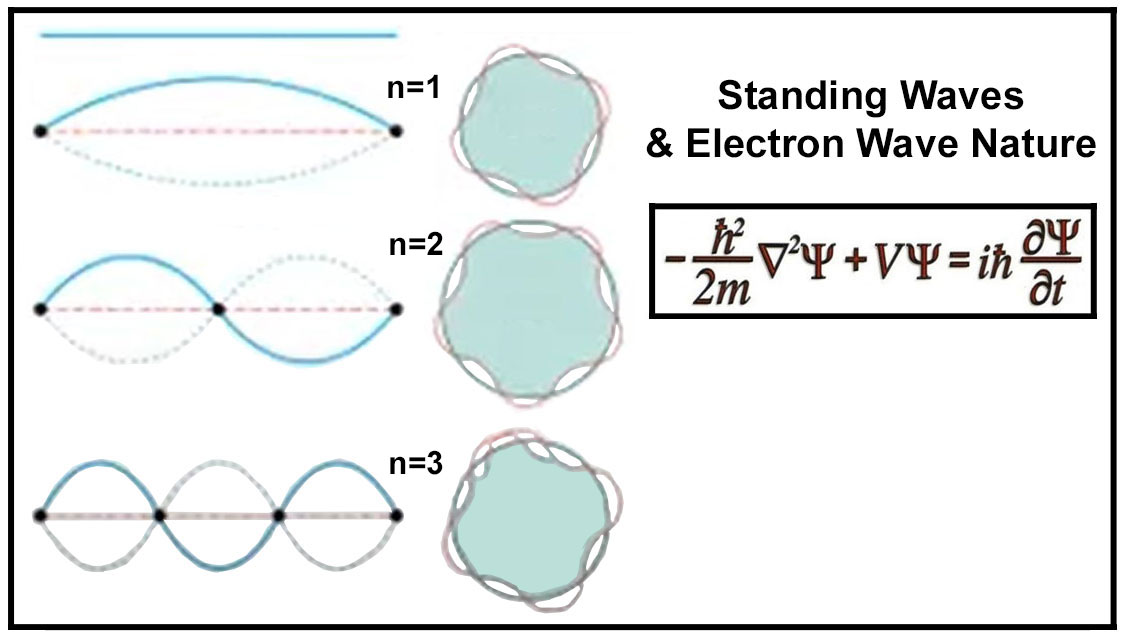
1926 - Schrodinger Equation
Erwin
Schrodinger published the paper "Quantization as an Eigenvalue Problem" ("Quantisierung als
Eigenwertproblem") now known as the Schrodinger Equation. It is a linear partial differential equation that
governs the wave function of a quantum-mechanical system and gives the correct energy eigenvalues for a
hydrogen-like atom. It is important to note that the Schrodinger Equation uses the
imaginary number i.
Electrons are wave-particle duality. Just like with a single string producing
multiple notes on a guitar an electron can exist in different number of harmonics. In physics, a standing wave,
also known as a stationary wave, is a wave which oscillates in time but whose peak amplitude profile does not move
in space. The locations at which the absolute value of the amplitude is minimum are called nodes, and the locations
where the absolute value of the amplitude is maximum are called antinodes. A standing wave must have whole
number repeats of 1/2 wavelengths. A standing wave must have whole number repeats of 1/2 wavelengths. Since
the electron is held fixed by the atractive force of the nucleus, it is similar to a standing wave whose ends are
also fixed (like the guitar string). A standing wave must have whole number repeats of 1/2
wavelengths.
Erwin Schrodinger developed a mathematical model where the electron was assumed to be
a standing wave. Schrodinger's paper has been universally celebrated as one of the most important
achievements of the twentieth century and created a revolution in quantum mechanics as well as physics and
chemistry in general. The philosophical issues raised by Schrodinger's
cat are still debated today and remain his most enduring legacy in popular science.
Schrodinger was awarded the Nobel Prize in Physics (1933).
1927 - Heisenberg's Uncertainty
Principle
Werner Heisenberg's (1901–1976) Uncertainty Principle asserts a fundamental
limit to the accuracy with which the values for certain pairs of physical quantities of a particle, such as
position and momentum can be predicted from initial conditions.

1927 - The Big
Bang
Astronomer Georges Lemaitre (1894-1966) first noted in 1927 that an expanding
universe could be traced back in time to an originating single point, which he called the "primeval atom".
Edwin Hubble
(1889–1953) confirmed through analysis of galactic redshifts in 1929 that galaxies were drifting apart - an
important observational evidence for an expanding universe. For several decades, the scientific community was
divided between supporters of the Big Bang and the rival steady-state model which stipulated an eternal universe
in contrast to the Big Bang's finite age. In 1965, the Cosmic Microwave Background (CMB) was discovered, which
convinced many cosmologists that the steady-state theory was falsified, since, unlike the steady-state theory,
the hot Big Bang predicted a uniform background radiation throughout the universe caused by the high
temperatures and densities in the distant past. A wide range of empirical evidence strongly favors the Big Bang,
which is now essentially universally accepted.
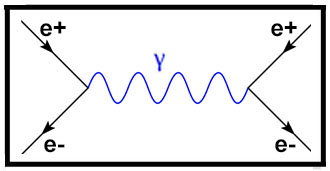
1928 – Dirac derives the Existence of
Antimatter
Theoretical physicist Paul Dirac (1902–1984) published a paper (1928) proposing that electrons can have both a
positive and negative charge. In 1931 Dirac predicted the existence of an as-yet-unobserved particle that he
called an "anti-electron" ('positron') that would have the same mass and the opposite charge as an electron and
that would mutually annihilate upon contact with an electron. In 1932 particle physicist Carl Anderson (1905-1991)
discovered the positron for which he won the Nobel Prized for Physics in
1936.

1929 – Hubble Confirms the Expansion of the
Universe
1912: Vesto Slipher (1875-1969) discovers that light from remote
galaxies was redshifted, indicating that galaxies were receding from the Earth.
1922: Using Einstein’s field
equations Alexander Friedmann used Einstein field equations to provide theoretical
evidence that the universe is expanding.
1927: Georges Lemaitre independently reached a similar conclusion to Friedmann on a theoretical basis, and also
presented the first observational evidence for a linear relationship between distance to galaxies and their
recessional velocity.
1929: Edwin Hubble observationally confirmed Lemaitre findings.
Assuming the cosmological principle, these findings would imply that all galaxies are moving away from each
other.
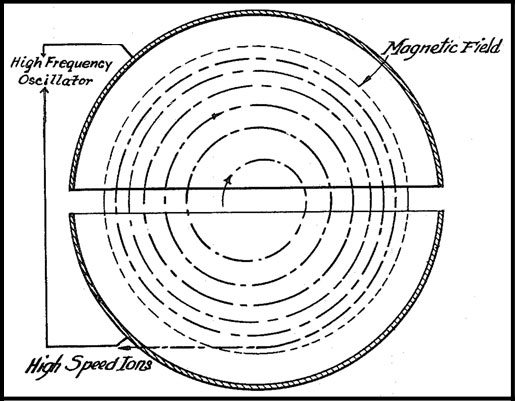
1930 - Cyclotron Developed
Ernest O. Lawrence (1901–1958) develops the cyclotron particle accelerator at the University of California,
Berkeley (patented in 1932). A cyclotron accelerates charged particles, via a static magnetic field and a varying
radio frequency, outwards from the center of a cylindrical vacuum chamber along a spiral path. The cyclotron was an
important improvement over linear accelerators (linacs) which provided higher energy particles, with a smaller
footprint and lower cost. Particle physicists were able to generate particle energies over 700MeV. In the 1950s,
the cyclotron was replaced with the synchrotron particle accelerator. The largest synchrotron-type accelerator is
the 27km circumference (17mi) Large Hadron Collider (near Geneva, Switzerland) which can accelerate beams of
protons to an energy of 6.5TeV. Lawrence was awarded the Nobel Prize in Physics (1939) for the invention and
development of the cyclotron and for the results obtained in regard to artificial radioactive
elements.
1932 – Chadwick
Discovers the Neutron
Cambridge physicist James Chadwick
(1891-1974) discovered a new type of “radiation” which was heavy enough to free protons from paraffin, but with no
charge. Chadwick showed that the new radiation was a neutral particle with the same mass as the proton.
Neutral proton or ‘neutron’. Neutrons and protons are known as nucleons. The nucleus is a hundred
thousand times smaller than an atom (few femtometers – 10-15 m). If the atom were scaled to the size of the
earth, the nucleus at the center would be just 10km wide, or the length of Manhattan. The nucleus harbors
practically all the mass of the atom in one tiny spot. The strong nuclear forces hold the protons and
neutrons together (which has to overcome the electrostatic repulsion of the neutron’s positive charges – inverse
square law). The strong force only appears at very small separations. In 1934, Hideki Yukawa proposed
that the nuclear force was carried by special particles called mesons. Protons and neutrons are glued
together by exchanging mesons. Chadwick was awarded the Noble Prize in Physics for the discovery of the neutron
(1935).
1933 – Zwicky
measure Dark Matter
Swiss astronomer Fritz Zwicky
(1898-1974) realized that a nearby giant cluster of galaxies was behaving in a way that implied it mass was much
greater than the weight of all the stars in all the galaxies within it. He inferred that some unknown dark
matter accounted for 400 times as much material as luminous matter, glowing stars and hot gas, across the entire
cluster. The sheer amount of dark matter was a big surprise, implying that most of the universe was not in
the form of stars and gas but something else.
Mass is also missing from individual spiral galaxies. Gas in the outer regions
rotates faster than it should if the galaxy was only as heavy as the combine mass of stars within it. So such
galaxies are more massive than expected by looking at the light alone. Again, the extra dark matter needs to
be hundreds of times more abundant than the visible stars and gass. Dark matter is not only spread throughout
galaxies but its mass is so great it dominates the motions of every star within them. Dark matter even extends
beyond the stars, filing a spherical “halo” or bubble around every flattened spiral galaxy disk.
Dark matter is made up of MACHOS or WiMPs
MACHOS – Massive Compact Halo Objects – dark gas clouds, dime stars or unlit
planets. In terms of relativity theory, the MACHO planets distort space-time, like a heavy ball depressing a
rubber sheet, which curves the light’s wavefront around it.
WIMPs – Weakly Interacting Massive Particles – shouldn’t have any effect on matter or
light. Difficult to detect. One candidate is the neutrino. Not enough neutrinos in the universe
to balance out the extra mass required. Suggest other exotic particles to be detected (axions,
photinos).

1937 - Discovery
of Superfluidity
Pyotr Kapitsa (1894-1984) discovered superfluidity in helium-4 where the viscosity drops to zero and the fluid
flows without any loss of kinetic energy. When stirred, a superfluid forms vortices than continue to rotate
indefinitely.
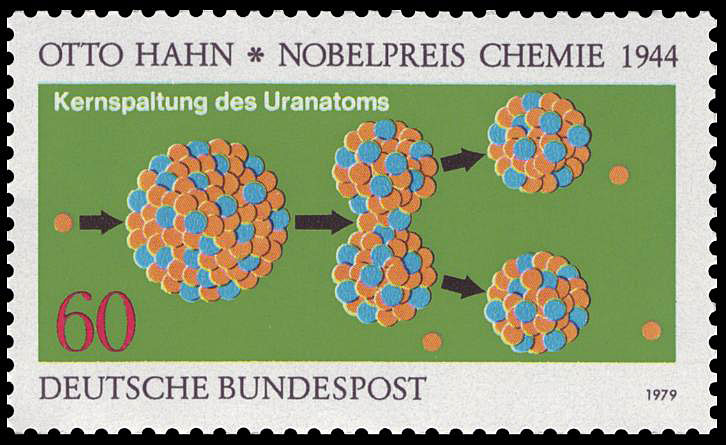
1938 – Atomic
Fission is Observed
German scientists Otto Hahn (1879-1968)
and Fritz Strassmann (1902-1980) shot neutrons into the heavy element uranium, attempting to create new
heavier metals. They got much lighter elements, some half the mass of uranium. It was like a watermelon
splitting in two when hit by a cherry. Colleagues Lise Meitner and Otto Frisch (living in Sweden during
fascist Germany) realized that energy would be released as the nucleus split because the two halves took up less
energy overall. Meitner and Frisch’s paper introduced the word “fission” after the division of a biological
cell. Later, Enrico Fermi obtained the first chain reaction in 1942 (University of Chicago, beneath the
football stadium). In 1967 Otto Frisch comments, “…gradually we came to the idea that perhaps one
should not think of the nucleus being cleaved in half as with a chisel, but rather that perhaps there was something
in Bohr’s idea that the nucleus was like a liquid drop.”
“The Structure of Scientific Revolutions”
(Thomas Kuhn, 1962): “One reason why that nuclear reaction proved especially difficult to
recognize was that men who knew what to expect when bombarding uranium chose chemical tests aimed mainly at
elements from the upper end of the periodic table. Paradigm procedures and applications are as necessary to science
as paradigm laws and theories and they have the same effects. Inevitably they restrict the phenomenological
field accessible for scientific investigation at any given time… The discovery of X-rays could seem to open a
strange new world to many scientists and could thus participate so effectively in the crisis that led to 20th
century physics.”
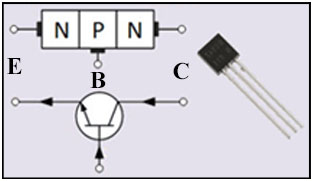
1948 - Development of the Transistor
John Bardeen (1908–1991), Walter Brattain (1902–1987) and William Shockley
develop point-contact (1947) and bipolar junction (1948) transistors at AT&T's Bell Labs (Murray Hill, NJ). The
transistor revolutionized the electronics industry, making possible the development of almost every modern
electronic device, from telephones to computers, and ushering in the Information Age. Shockley, Bardeen, and
Brattain were jointly awarded the 1956 Nobel Prize in Physics.
1956 – Neutrinos are Detected
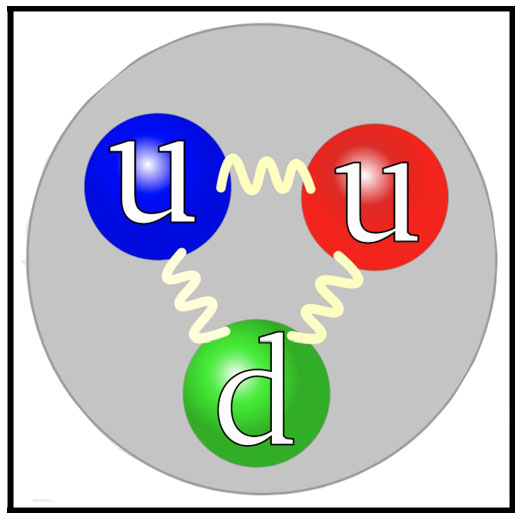
1964 - Quark Model
The
quark model was independently proposed by physicists Murray Gell-Mann (1929–2019) and George Zweig in 1964.
Quarks were introduced as parts of an ordering scheme for hadrons, and there was little evidence for their
physical existence until deep inelastic scattering experiments at the Stanford Linear Accelerator Center
(1968) where electrons were scattering from each other more widely than calculations suggested — indicating
that protons and neutrons were made of even smaller particles. The name "quark" was coined by Gell-Mann who
borrowed the line "Three quarks for Muster Mark!" (the hypothetical particles came in threes) from the James
Joyce's novel Finnegans Wake.
According to physicists, quarks first appeared 10-12 seconds after the Big Bang when two of four fundamental forces
(the weak force and the electromagnetic force) separated. The antiparticles of quarks, or antiquarks, also
appeared around this time. There are six different “flavors” of quarks: up, down, strange, charm, bottom, and
top, each with different masses and charges. Quarks can never be seen alone due to a property known as color
confinement. The energy required to remove a quark from a proton or separate two quarks immediately produces
an antiquark, which quickly turns a single quark back into a hadron. Computer models have to be used to
determine their mass by simulating the interaction between quarks and gluons — the particles that glue quarks
together.
1965 – Cosmic Microwave Background Discovered by Arno
Penzias (1933-2024) and Robert Wilson
1965 - Quantum Electrodynamics
In particle physics, quantum electrodynamics (QED) is the relativistic quantum field theory of
electrodynamics. In essence, it describes how light and matter interact and is the first theory where full
agreement between quantum mechanics and special relativity is achieved. Shin'ichirō Tomonaga (1906-1979),
Julian Schwinger (1918-1994), Richard Feynman (1918–1988) and Freeman Dyson (1923–2020) produced fully
covariant formulations that were finite at any order in a perturbation series of quantum electrodynamics.
Tomonaga, Schwinger, and Feynman were jointly awarded the 1965 Nobel Prize in
Physics.
1980 – Quantum Computing
Theoretical physicist Richard Feynman and mathematician Yuri Manin (1937–2023) suggested that a
quantum computer had the potential to simulate things a classical computer could not. Quantum computing is
the exploitation of collective properties of quantum states, such as superposition and entanglement, to
perform fast, complex computation such as integer factorization for RSA encryption. In 1994, Peter Shor
developed a quantum algorithm for factoring integers with the potential to decrypt RSA-encrypted
communications. Quantum computing is likely to find applications in pharmaceutical, biomedicine, data
security, machine learning, autonomous vehicle systems and other applications.
1998 – Supernova Data suggests Dark
Energy
21st CENTURY
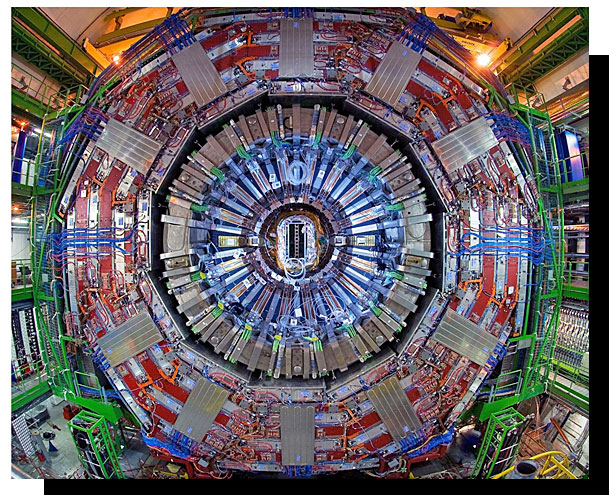
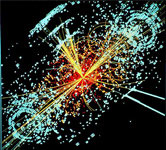
2012 - Higgs Boson Particle
Detected
The Higgs boson (the ‘God
Particle’) is the fundamental particle associated with the Higgs field, a field that gives mass to other
fundamental particles such as electrons and quarks. A particle’s mass determines how much it resists changing
its speed or position when it encounters a force. Not all fundamental particles have mass. The photon, which
is the particle of light and carries the electromagnetic force, has no mass at all. The Higgs boson was
proposed in 1964 by Peter Higgs, François Englert, and four other theorists to explain why certain particles
have mass. Scientists confirmed its existence in 2012 through the ATLAS (A Toroidal LHC Apparatus) and CMS
(Compact Muon Solenoid) experiments at the Large Hadron Collider (LHC) at CERN in Switzerland. The CMS
detector is built around a huge solenoid magnet. This takes the form of a cylindrical coil of superconducting
cable that generates a field of 4 tesla, about 100,000 times the magnetic field of the Earth. The field is
confined by a steel “yoke” that forms the bulk of the detector’s 14,000-tonne weight. This discovery led to
the 2013 Nobel Prize in Physics being awarded to Higgs and Englert.
Sidenote: as of AUG20 mysteries remain, such as why particles have
different masses. To answer the 'mysteries' more precise measurements are necessary and the need to build a
more powerful collider.
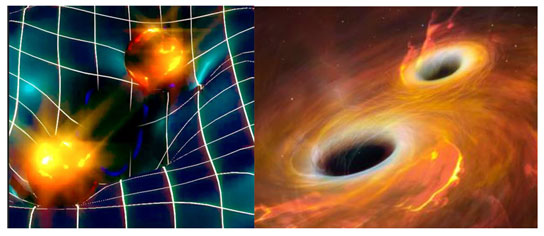
2015 - Gravitational Waves
Detected
The first direct observation of gravitational waves was made in 2015, when a
signal generated by the merger of two black holes was received by the LIGO (Laser Interferometer
Gravitational-Wave Observatory) gravitational wave detectors in Livingston and in Hanford. The 2017 Nobel
Prize in Physics was subsequently awarded to Rainer Weiss, Kip Thorne and Barry Barish for their role in the
direct detection of gravitational waves.
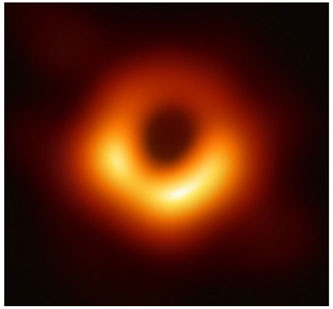
2019 - 1st Image of a Black
Hole
Using the Event Horizon Telescope,
scientists obtained an image of the black hole at the center of the galaxy M87. Messier 87 (also known as
Virgo A or NGC 4486, generally abbreviated to M87) is a supergiant elliptical galaxy with several trillion
stars in the constellation Virgo. M87 is about 16.4 million parsecs (53 million light-years) from Earth and
is the second-brightest galaxy within the northern Virgo Cluster, having many satellite galaxies. One of the
most massive galaxies in the local universe, it has a large population of globular clusters—about 15,000
compared with the 150–200 orbiting the Milky Way—and a jet of energetic plasma that originates at the core
and extends at least 1,500 parsecs (4,900 light-years), traveling at a relativistic speed. It is one of the
brightest radio sources in the sky and a popular target for both amateur and professional
astronomers.
| 










































